Information
- Publication Type: Master Thesis
- Workgroup(s)/Project(s):
- Date: March 2021
- Date (Start): 2. July 2020
- Date (End): 10. March 2021
- TU Wien Library:
- Diploma Examination: 10. March 2021
- Open Access: yes
- First Supervisor: Eduard Gröller

Abstract
During the treatment process of a patient the physician usually requests a Laboratory Report (e.g. a blood count) from the laboratory. The delivery of the Laboratory Report is ususally performed via fax or letter to the treating physician. The structured laboratory data, which were initially generated by the laboratory, are not available for the physician. Furthermore, the physician has to import the Laboratory Report manually to the Electronic Medical Record (EMR) system. Thus, enabling the electronic data exchange between a laboratory and relevant healthcare providers improves the current treatment processes.
The aim was the connection between a laboratory and an existing distributed Health Information Exchange (HIE), where several healthcare providers are connected to exchange medical docu-ments via the Cross-Enterprise Document Sharing (XDS) profile. A challenge was to perform the integration transparently with existing established exchange mechanisms and interfaces. While the Laboratory Information System (LIS) sends laboratory data via Health Level 7 (HL7) V2 messages over Transmission Control Protocol/Internet Protocol (TCP/IP), the HIE follows the document-based approach, and exchanges documents via XDS transactions over SOAP 1.2.
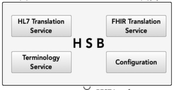
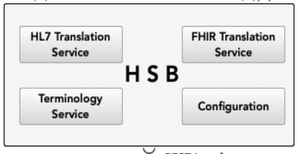
A Clinical Data Repository (CDR) has been established for the storage and management of the laboratory data as Fast Healthcare Interoperability Resources (FHIR) resources. Furthermore, a Health Service Bus (HSB) has been developed to support the communication between the LIS, the CDR, and the HIE participating systems and components. The Clinical Document Architecture (CDA) standard was used to create a structured laboratory document, which has been exchanged with the participating healthcare providers of the HIE. The HSB integrates translation engines, which are responsible for the mapping from HL7 V2 messages into FHIR resources and further from FHIR resources into CDA documents.
The integration of the laboratory with the HIE was successful. An adequate mapping between the HL7 V2, FHIR, and CDA standards has been specified. Gaps between the particular standards have been identified and if necessary, an extension of the data structure has been defined. FHIR has proven its suitability as a flexible and robust storage format and its ability to provide the appropriate data structure to map laboratory data from HL7 V2 and convert FHIR resources to a CDA document.
Additional Files and Images
Additional images and videos
Additional files
Weblinks
No further information available.
BibTeX
@mastersthesis{schmidlehner2021,
title = "Standards-based Clinical Data Repository",
author = "Sandra Schmidlehner",
year = "2021",
abstract = "During the treatment process of a patient the physician
usually requests a Laboratory Report (e.g. a blood count)
from the laboratory. The delivery of the Laboratory Report
is ususally performed via fax or letter to the treating
physician. The structured laboratory data, which were
initially generated by the laboratory, are not available for
the physician. Furthermore, the physician has to import the
Laboratory Report manually to the Electronic Medical Record
(EMR) system. Thus, enabling the electronic data exchange
between a laboratory and relevant healthcare providers
improves the current treatment processes. The aim was the
connection between a laboratory and an existing distributed
Health Information Exchange (HIE), where several healthcare
providers are connected to exchange medical docu-ments via
the Cross-Enterprise Document Sharing (XDS) profile. A
challenge was to perform the integration transparently with
existing established exchange mechanisms and interfaces.
While the Laboratory Information System (LIS) sends
laboratory data via Health Level 7 (HL7) V2 messages over
Transmission Control Protocol/Internet Protocol (TCP/IP),
the HIE follows the document-based approach, and exchanges
documents via XDS transactions over SOAP 1.2. A Clinical
Data Repository (CDR) has been established for the storage
and management of the laboratory data as Fast Healthcare
Interoperability Resources (FHIR) resources. Furthermore, a
Health Service Bus (HSB) has been developed to support the
communication between the LIS, the CDR, and the HIE
participating systems and components. The Clinical Document
Architecture (CDA) standard was used to create a structured
laboratory document, which has been exchanged with the
participating healthcare providers of the HIE. The HSB
integrates translation engines, which are responsible for
the mapping from HL7 V2 messages into FHIR resources and
further from FHIR resources into CDA documents. The
integration of the laboratory with the HIE was successful.
An adequate mapping between the HL7 V2, FHIR, and CDA
standards has been specified. Gaps between the particular
standards have been identified and if necessary, an
extension of the data structure has been defined. FHIR has
proven its suitability as a flexible and robust storage
format and its ability to provide the appropriate data
structure to map laboratory data from HL7 V2 and convert
FHIR resources to a CDA document.",
month = mar,
address = "Favoritenstrasse 9-11/E193-02, A-1040 Vienna, Austria",
school = "Research Unit of Computer Graphics, Institute of Visual
Computing and Human-Centered Technology, Faculty of
Informatics, TU Wien",
URL = "https://www.cg.tuwien.ac.at/research/publications/2021/schmidlehner2021/",
}
 image
image Master Thesis
Master Thesis Poster
Poster